The extension of the European Regulation 2017/745 (MDR) was published in the Official Journal
With the publication in the Official Journal of the EU (number L080), the extension of the European Regulation 2017/745 (MDR) came into force. The amendment now applies to the entire Union, as stated in Article 1 of the MDR and IVDR.
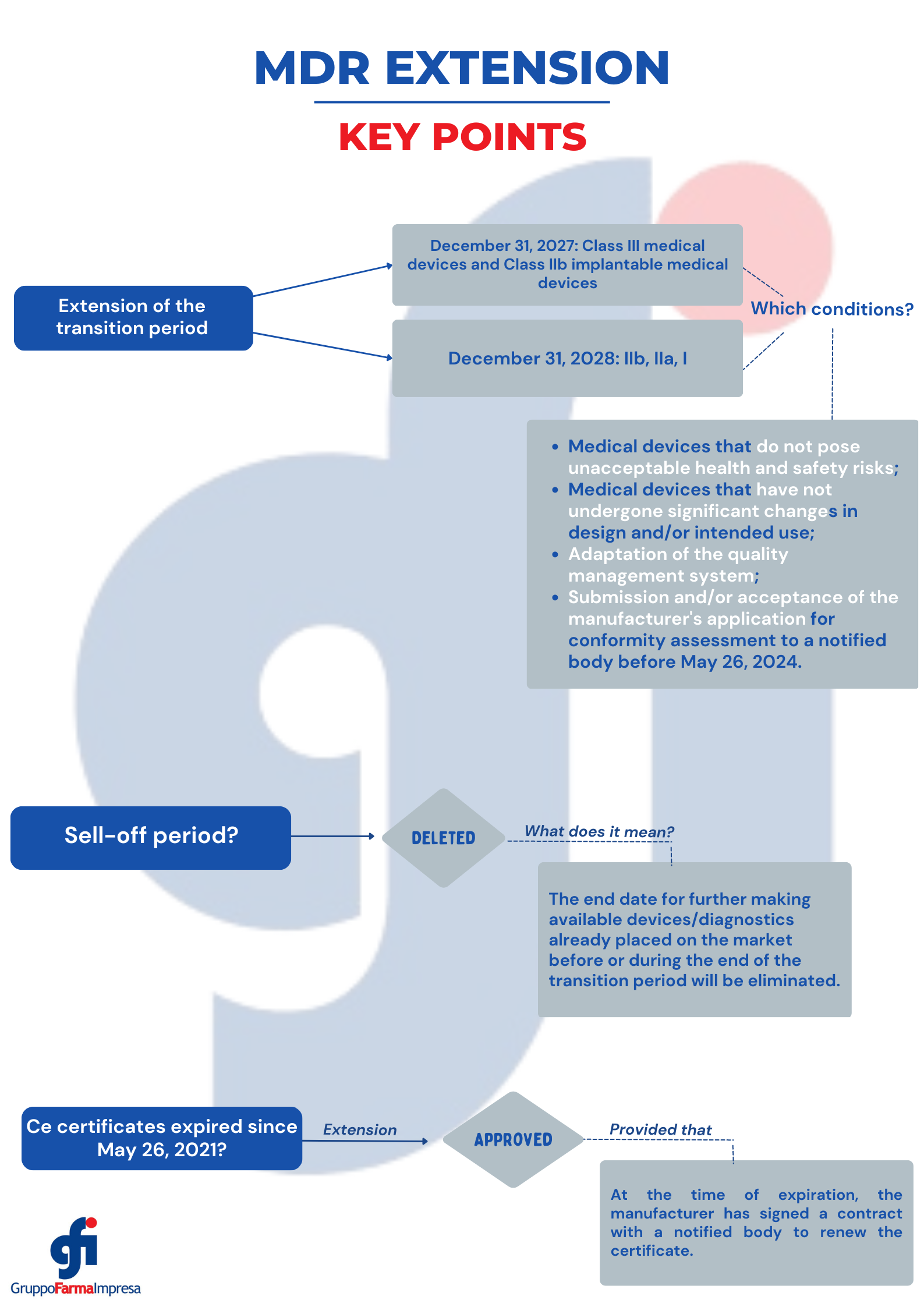
1. Extension of the transition period, depending on the risk class of the device, to be determined according to the MDR classification rules.
- Dec. 31, 2027: for class III medical devices and class IIb implantable medical devices; except sutures, staples, dental fillings, orthodontic appliances, screws, wedges, plates, wires, pins, clips and connectors;
- Dec. 31, 2028: Class IIb (other than those mentioned above), IIa and I devices.
The extension is subject to several conditions such that only safe devices, for which the manufacturer has submitted an MDR application for conformity assessment by May 26, 2024, benefit from the extended transition period.
2. Extension of the validity of certificates, provided the conditions for the extended transition period are met. Certificates that have already expired after May 26, 2021 may also be considered valid if additional conditions are met.
3. Transfer of appropriate oversight to MDR notified bodies by September 26, 2024.
4. Introduction of a temporary waiver until May 26, 2026 to the quality management system certificate requirement for class III custom-made implantable devices under certain conditions.
5. For both medical devices and in vitro diagnostics, the sell-off period, i.e., the end date for further making available devices/diagnostics already placed on the market before or during the end of the transition period, will be deleted. This would eliminate unnecessary disposal of safe devices/diagnostics already placed on the market.
It is important for importers and distributors to realize that the amendment radically changes the concept of a “legacy device“ to one that complies with the new Article 120,MDR.
How can I prove to others that my certificate is actually valid?
Although the operation of extending or revitalizing certificate duration is automatic, this does not mean that it will automatically result in a new or amended certificate. In fact, the amendment does not provide a mechanism for this. The method by which it will be possible to prove to the rest of the world that one’s expired certificate is indeed still valid has not yet been well defined. This point may be clarified in the Q&A guide due to be published this week.
The European Commission is already a step ahead, discussing the implementation of the amendment and the future of the EU’s medical device regime, which is still in serious trouble. According to a Commission briefing note for the March 14 EPSCO Council, the MDR is not yet in the safe zone and the amendment and introduction of MDCG 2022-14 will require everyone’s full attention and participation.
This briefing note from the Commission is important for its forward-looking part and for some past conclusions. The Commission believes it is important to address a number of structural issues before the end of the extended transition periods:
- issues related to orphan devices;
- access of small manufacturers to notified bodies;
- duration and cost of conformity assessment procedures;
- the interaction between clinical trials for medicines and performance evaluation studies for in vitro diagnostics.
The Commission explicitly states that these problems have appeared in the current regulatory framework and have a negative impact on patient safety, public health, and medical innovation, which is a great admission of how the Commission views the success of the MDR so far and what are the causes of the MDR’s shortcomings that have led to unsatisfactory results. It also shows that the Commission does not seem to believe that the current actions on some of these points are actually resolving them.
The Commission will gather further evidence for the comprehensive evaluation of the MDR and IVDR scheduled for May 2027 (Article 121 MDR and Article 111 IVDR).
If your company is looking for an MDR-certified medical device
in ready to market line and contract manufacturing
useful in the treatment of Dyspeptic Disorders, Gastrointestinal Bloating, Aerophagy and Gas Colic,